
 Department
of Crystal and Structural Chemistry
Department
of Crystal and Structural Chemistry
Padualaan 8
(H.R. Kruytgebouw), 3584 CH Utrecht, the Netherlands
phone: +31-30-2533502
(secr.) fax: +31-30-2533940, e-mail: secrks@chem.uu.nl
Research group of Eric Huizinga

 Department
of Crystal and Structural Chemistry
Department
of Crystal and Structural Chemistry
Padualaan 8
(H.R. Kruytgebouw), 3584 CH Utrecht, the Netherlands
phone: +31-30-2533502
(secr.) fax: +31-30-2533940, e-mail: secrks@chem.uu.nl
Research group of Eric Huizinga

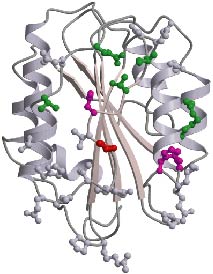 Ribbon
drawing of the crystal structure of the VWF-A3 domain. The color of residues
shown in ball-and-stick reflects the effect of their mutation to alanine
on collagen binding (red/magenta abolish collagen binding; green decreased
binding; grey no effect). The shape and location of the collagen binding
site of VWF-A3 is strikingly different from collagen binding sites found
in homologous integrin I-type domains. VWF-A3 has a rather flat binding
site in one of its side faces, while integrin I-domains have a groove shape
binding site located at their top-face.
Ribbon
drawing of the crystal structure of the VWF-A3 domain. The color of residues
shown in ball-and-stick reflects the effect of their mutation to alanine
on collagen binding (red/magenta abolish collagen binding; green decreased
binding; grey no effect). The shape and location of the collagen binding
site of VWF-A3 is strikingly different from collagen binding sites found
in homologous integrin I-type domains. VWF-A3 has a rather flat binding
site in one of its side faces, while integrin I-domains have a groove shape
binding site located at their top-face.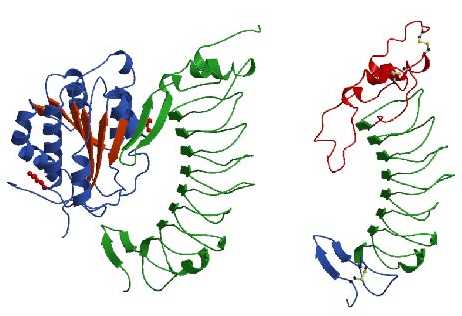 On
the left: Ribbon drawing of the A1 domain of VWF (blue and red) bound to
the VWF-binding domain of GpIb-alpha (green). Shown in red ball-and-stick
are two mutated residues, R543Q in VWF-A1 and M239V in GpIb-alpha that
enhance complex formation and cause von Willebrands disease. We introduced
these mutations to obtain a strong complex for crystallisation. Upon complex
formation a surface exposed loop, called beta-switch, that is disordered
in the structure of free GpIb-alpha (shown on the right) changes its conformation
to a beta-hairpin and aligns with the central beta-sheet of VWF-A1. Mutation
239V is located in this loop region and likely stabilizes the hairpin conformation
thereby enhancing the affinity of GpIb-alpha for VWF. The R543Q mutation
is located at the base of VWF-A1 and apparently destabilizes the
conformation of its N- and C-terminal peptides that may shield the GpIb-alpha
binding site in a low affinity conformation of VWF. Under physiological
conditions displacement of the terminal peptides may result from a pulling
force created by shear stress acting on VWF immobilized onto collagen thereby
providing activation of platelet adhesion.
On
the left: Ribbon drawing of the A1 domain of VWF (blue and red) bound to
the VWF-binding domain of GpIb-alpha (green). Shown in red ball-and-stick
are two mutated residues, R543Q in VWF-A1 and M239V in GpIb-alpha that
enhance complex formation and cause von Willebrands disease. We introduced
these mutations to obtain a strong complex for crystallisation. Upon complex
formation a surface exposed loop, called beta-switch, that is disordered
in the structure of free GpIb-alpha (shown on the right) changes its conformation
to a beta-hairpin and aligns with the central beta-sheet of VWF-A1. Mutation
239V is located in this loop region and likely stabilizes the hairpin conformation
thereby enhancing the affinity of GpIb-alpha for VWF. The R543Q mutation
is located at the base of VWF-A1 and apparently destabilizes the
conformation of its N- and C-terminal peptides that may shield the GpIb-alpha
binding site in a low affinity conformation of VWF. Under physiological
conditions displacement of the terminal peptides may result from a pulling
force created by shear stress acting on VWF immobilized onto collagen thereby
providing activation of platelet adhesion.