Membrane Proteins
Research Goals
In our research, we determine the structure and function of medically-relevant membrane proteins.
Tetraspanins are ubiquitous membrane proteins that act as molecular organizers of eukaryotic plasma membranes by assembling specific partner proteins into so-called tetraspanin-enriched microdomains (TEMs). We studied the molecular basis for the interaction of tetraspanins with partner proteins using the tetraspanin CD9 and its prototypical partner, the single-span transmembrane protein EWI-F.
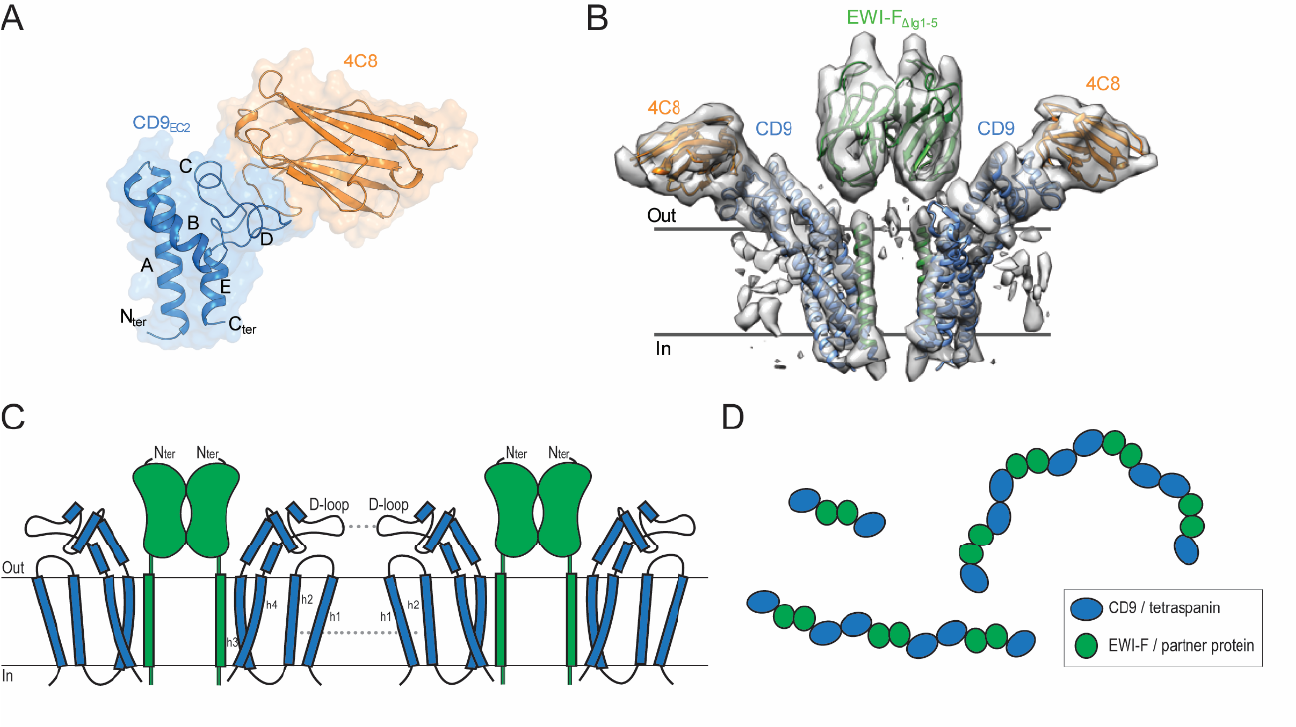
We elucidated high-resolution crystal structures of the second extracellular domain (EC2) of CD9 in complex with nanobodies 4C8 and 4E8 (panel A). We then used nanobody 4C8 to solve the cryo-EM structure of full-length CD9 in complex with extracellularly truncated EWI-F (panel B). The structure revealed that the CD9–EWI-F complex arranges as a hetero-tetramer, with a central EWI-F dimer flanked by a CD9 molecule on each side. The interaction between CD9 and EWI-F is mediated by the transmembrane domains of both proteins; membrane helices h3 and h4 of CD9 bind to the membrane helix of EWI-F. The D-loop of the EC2 of CD9 is bound by nanobody 4C8 and orients away from the complex with EWI-F. Notably, previous biochemical data have established that the D-loop represents a homo- and hetero-dimerization site in tetraspanins. Therefore, the CD9–EWI-F arrangement observed in the cryo-EM data is consistent with the formation of higher-order protein assemblies through a ‘concatenation model’, which may explain the occurrence of tetraspanin-enriched microdomains (panel C, D).
Six-transmembrane epithelial antigen of the prostate (STEAP) proteins, are a class of metalloreductase enzymes that is upregulated on the cell membranes of several human cancers. STEAPs catalyze the reduction of iron(III) and copper(II), which is a crucial process for the uptake of these metals by mammalian cells.
Molecular mechanisms of metal-ion reduction by human STEAP4
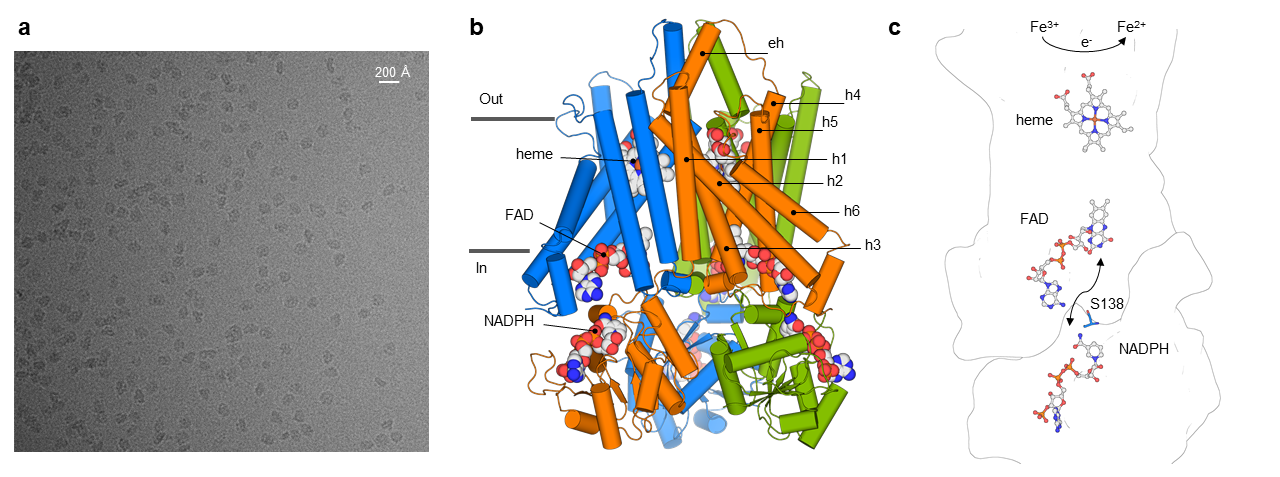
STEAPs contain a N-terminal intracellular oxidoreductase domain (OxRD) followed by a six-helical transmembrane domain (TMD). Using single-particle cryo-electron microscopy (panel a), we determined structures of human STEAP4 in the presence of its redox cofactors (NADPH, FAD, heme) and substrate (Fe3+-NTA), which revealed that STEAP4 possesses a trimeric, domain-swapped architecture (panel b). The observed linear arrangement of cofactors (panel c) defines the structural basis for how STEAPs transfer electrons across the membrane to metal-complexes at the membrane luminal side.
Insights into the structure and potential cellular function of human STEAP1
Next we focused on another STEAP-family member, STEAP1. Contrary to the other STEAP proteins, STEAP1 lacks an intracellular NADPH-binding domain and does not display cellular metalloreductase activity. However, STEAP1 is highly upregulated in various cancers and therefore represents a promising therapeutic target.
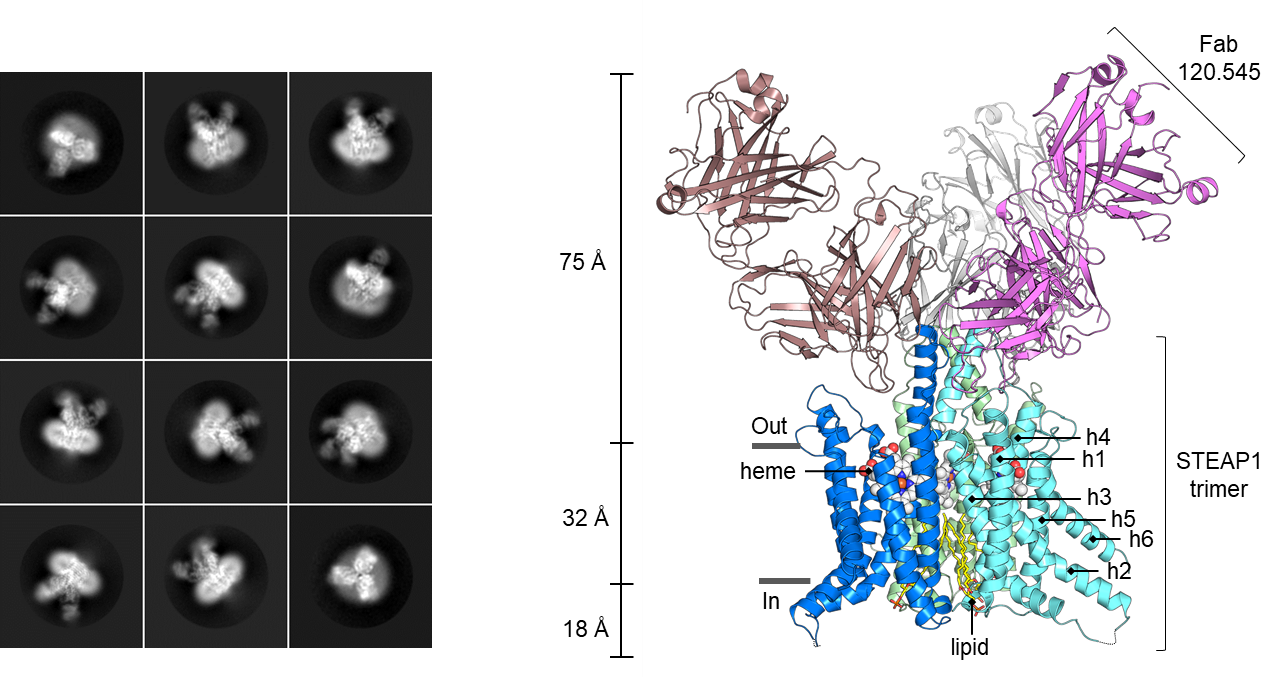
We solved the single-particle cryo-EM structure of trimeric human STEAP1 bound to antigen-binding fragments of the therapeutic antibody mAb120.545. The architecture of STEAP1 resembles that of STEAP4, indicating that STEAP1 could function as a metalloreductase when it is supplied with an electron-donating substrate like NADPH. We showed that a fusion protein composed of the intracellular NADPH-binding domain of STEAP4 and the transmembrane domain of STEAP1 is capable of reducing Fe3+ to Fe2+. These data suggest that STEAP1 could be a functional reductase in STEAP-heterotrimers. mAb120.545, of which humanized versions are used as antibody-drug conjugates in prostate cancer clinical trials, inhibits the cellular metalloreductase activity of the STEAP4/STEAP1 fusion protein, suggesting that antibodies can be used as general tools to inhibit the activity of STEAP enzymes.
Structural similarities between STEAPs and other transmembrane oxidoreductases
In collaboration with the lab of Prof. Andrea Mattevi at the University of Pavia in Italy, we performed a detailed structural comparison between STEAPs and the distantly homologues NADPH Oxidases (NOXs). NOX enzymes reduce extracellular di-oxygen to form reactive-oxygen species, and the first NOX structure (of C. stagnale NOX5) was solved by the Mattevi lab. We discovered that STEAP and NOX enzymes contain a highly similar four-helical bundle that binds two membrane-embedded redox cofactors to promote transmembrane electron transport. We then expanded our structural analysis to all structures in the pdb to reveal that the four helical cofactor-binding bundle is present in several transmembrane oxidoreductase enzymes, some of which were never related to STEAPs and NOXs before. Our analysis provides insights into the structure, mechanism and evolution of oxidoreductases that perform transmembrane electron transport.
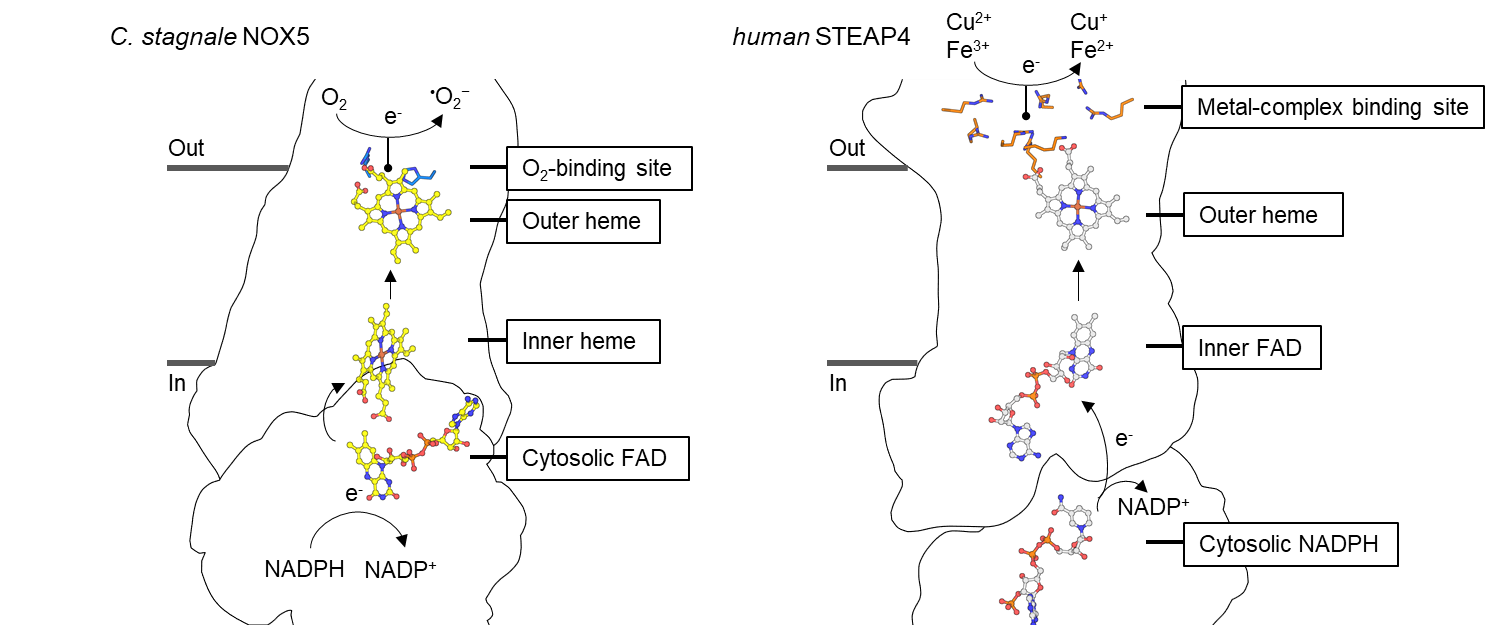
Comparison of the transmembrane arrangement of redox cofactors in NOX (Left) and STEAP (Right) families of oxidoreductases.