Piet Gros Lab
Bijvoet Centre for Biomolecular Research
Department of Chemistry, Faculty of Science
Utrecht University
email: p.grosuu.nl

Research Areas
Overview
Complement System
Plasma & Membrane Proteins
Crystallographic Method Development
Major Techniques
X-ray Crystallography
Cryo-Electron Microscopy
Biophysical Characterisation
Research Highlights
Tetraspanin CD9/EWI-F
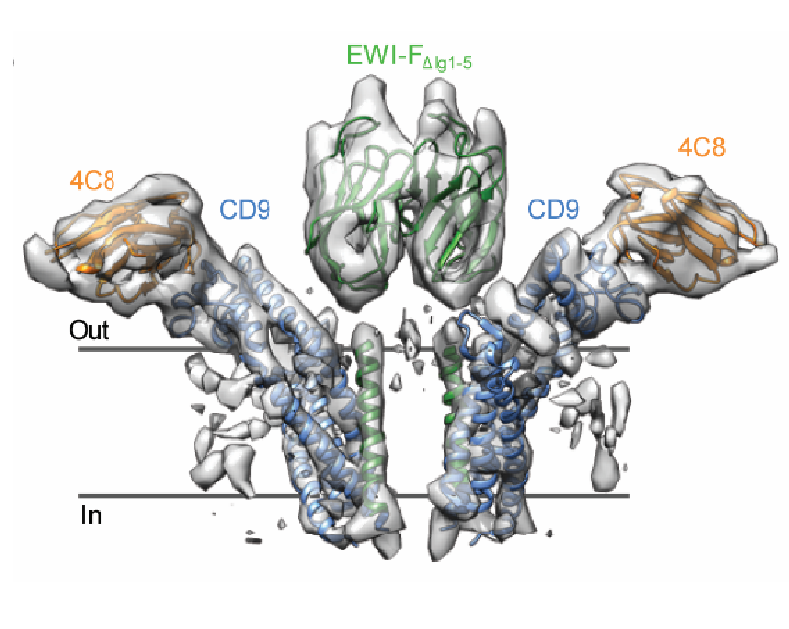
Cryo-EM structure of CD9 in complex with EWI-F; implications for Tetraspanin-enriched microdomain formation .
More...More STEAPs
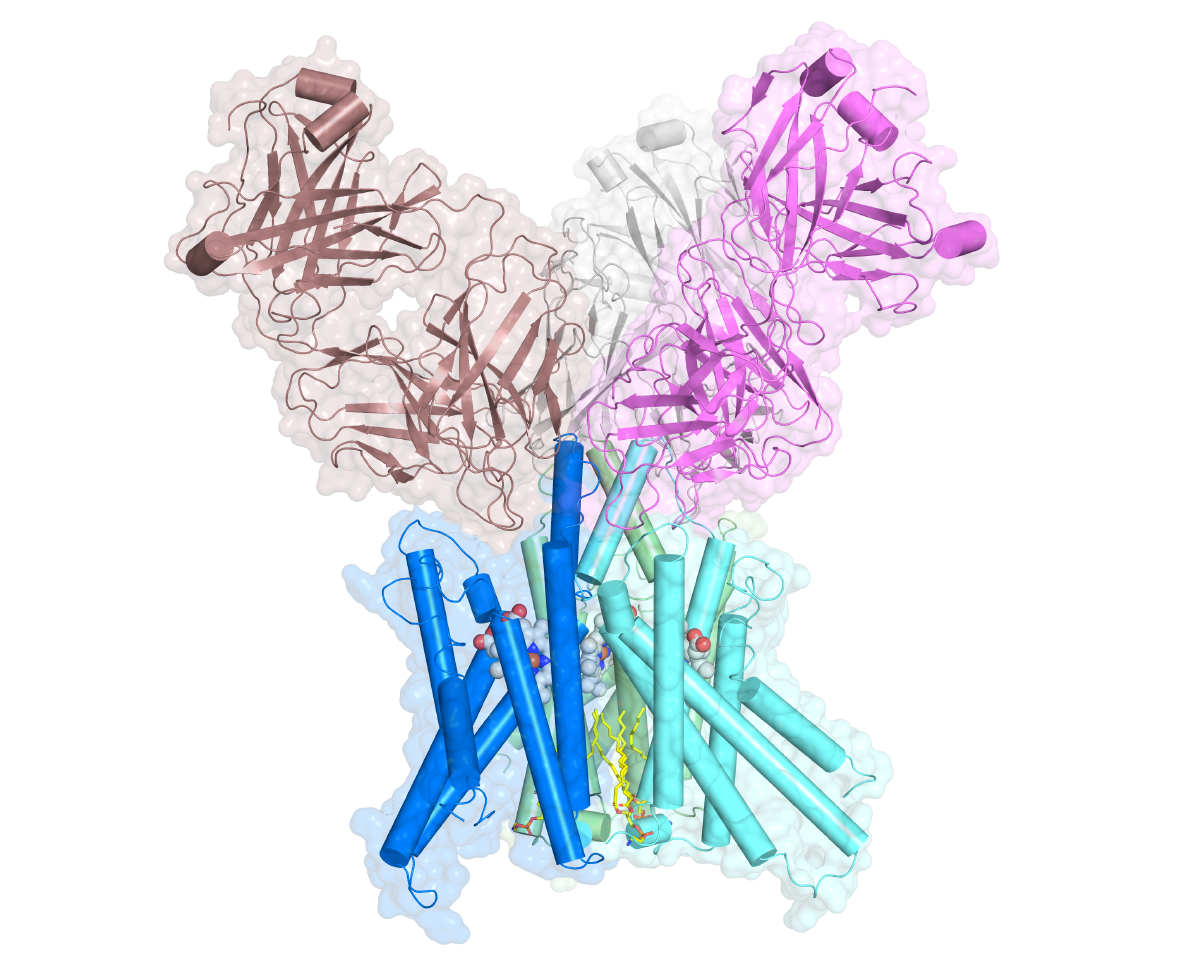
Structural and biochemical studies of STEAP1 suggest a role as modulator of iron(III) reduction.
More...Oligomeric Properdin
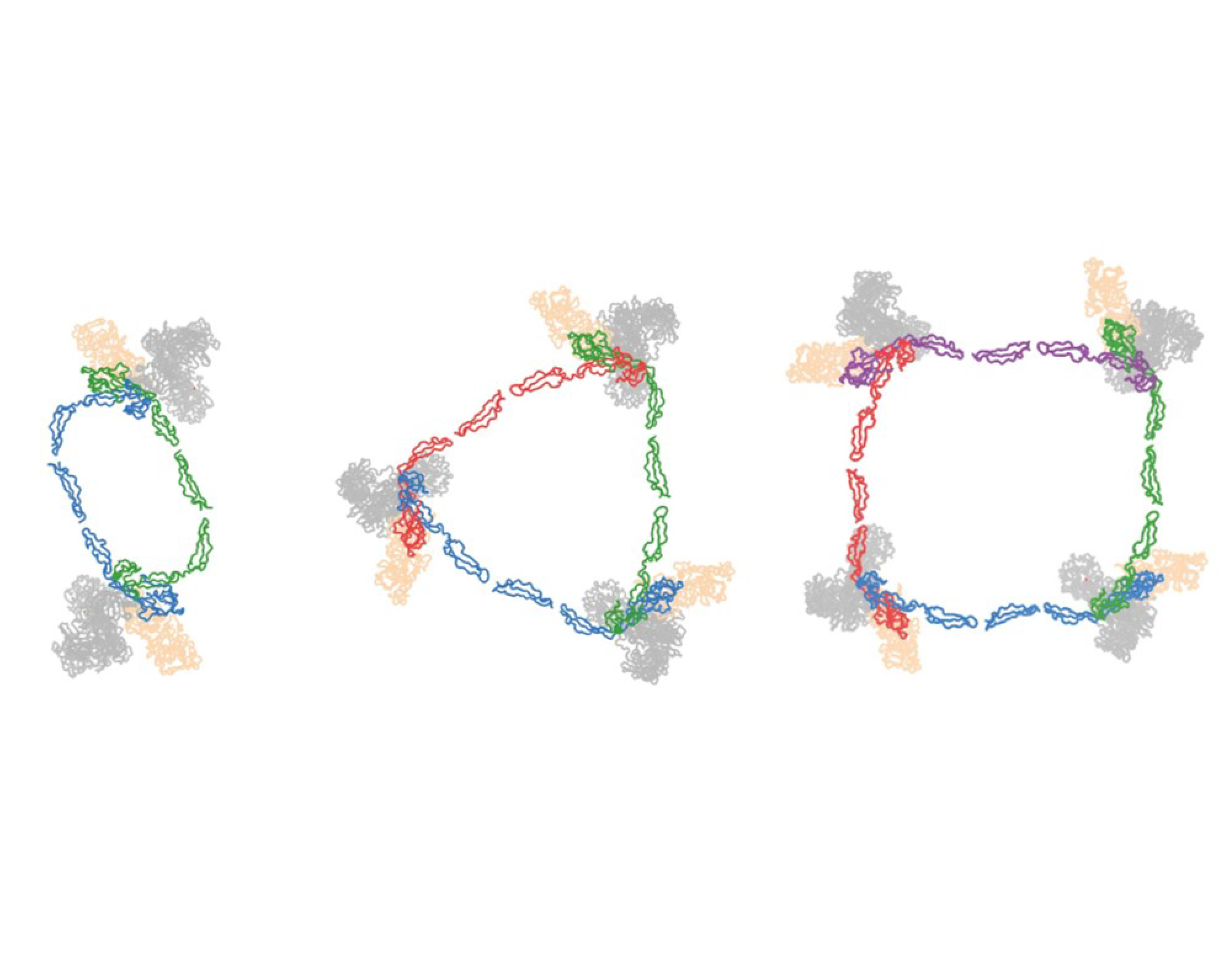
Complement enhancement by oligomeric properdin explained by crystal structures of properdin and properdin/C3b-CTC complexes.
More...Caught in the act
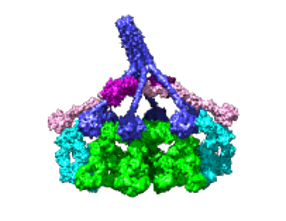
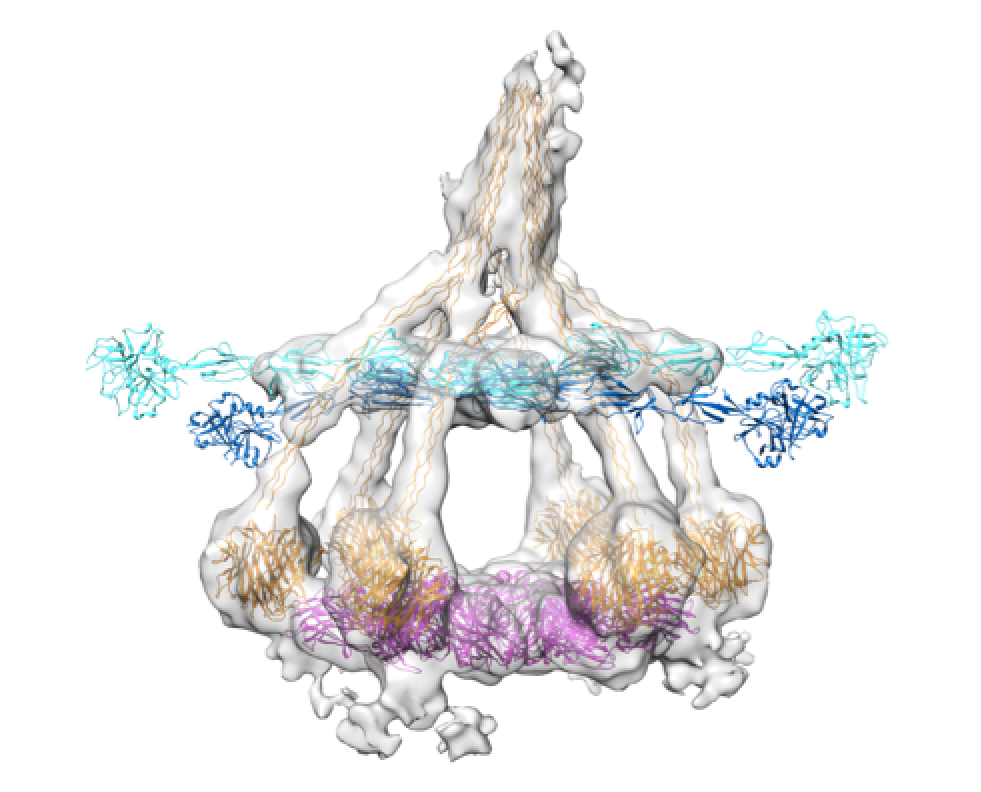
Structures of IgM-C1-C4b complexes on liposomes revealed by cryo-EM tomography.
More...Metal Homeostasis
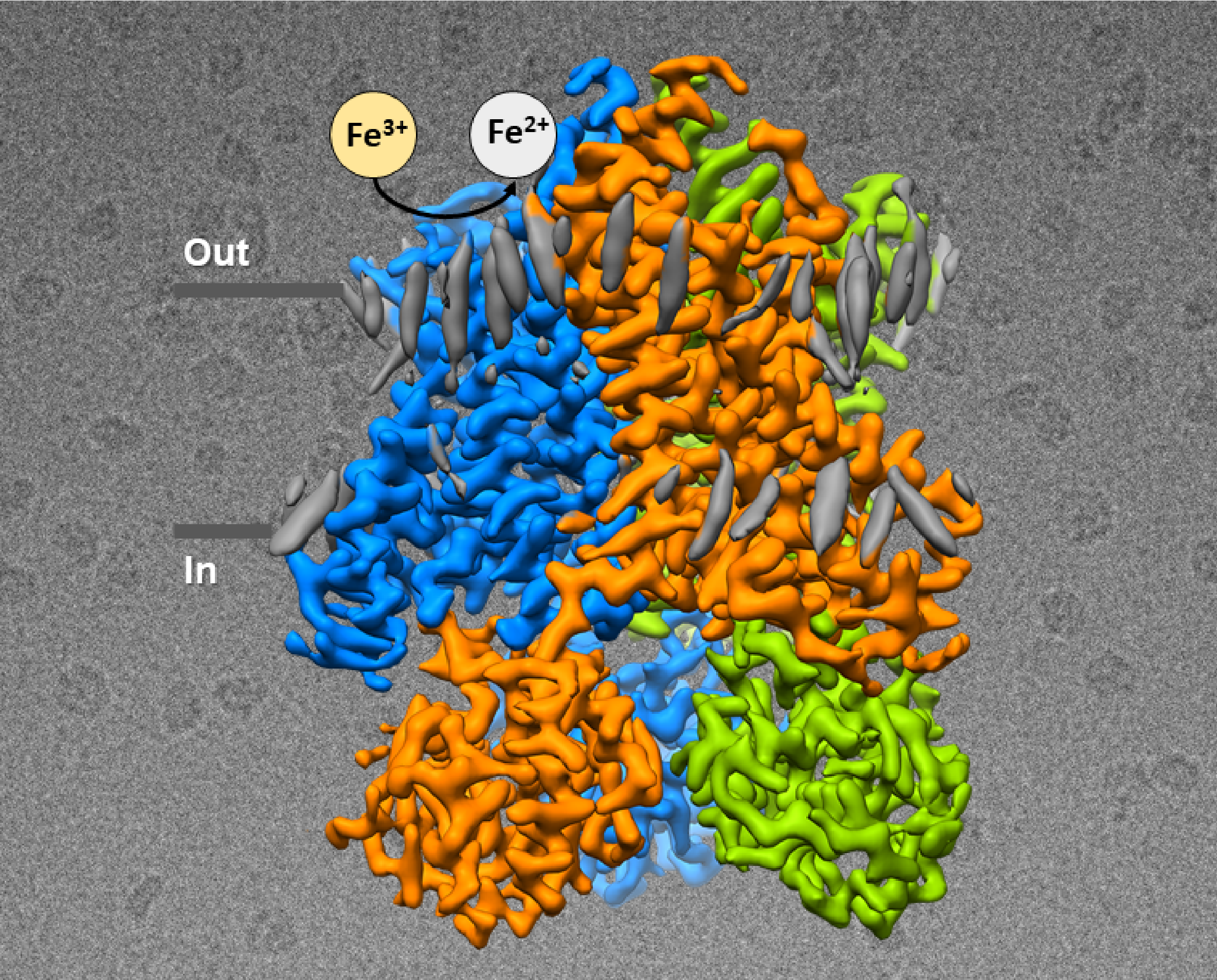
Cryo-EM structures of human STEAP4 reveal mechanism of iron(III) reduction.
More...Complement Regulation
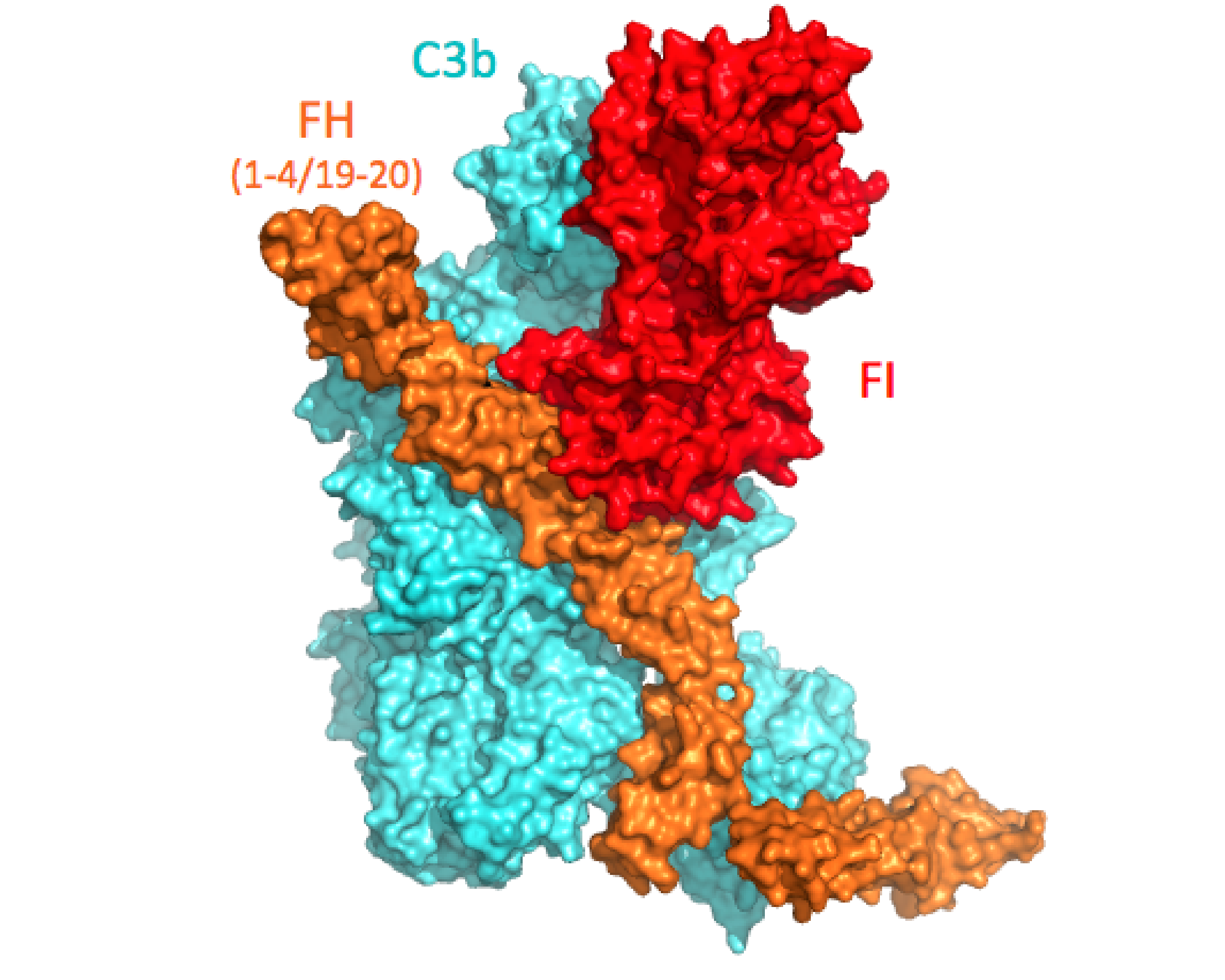
Ternary complex C3b-FH-FI reveals molecular mechanisms underlying co-factor activity and complement-immune processing
More...Complement Lysis
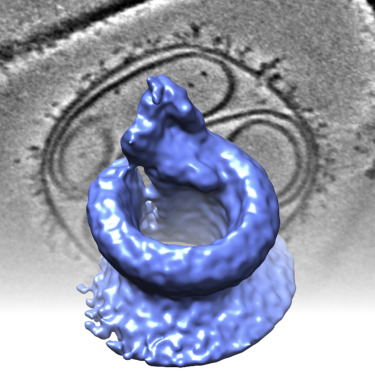
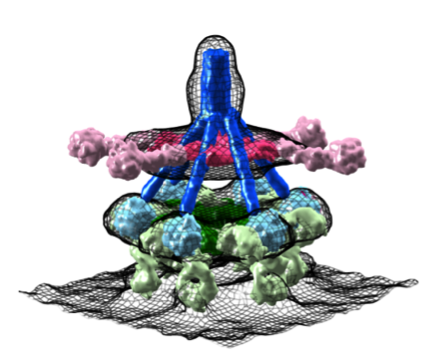
Heterogeneous MAC initiator and pore structures in a lipid bilayer by phase-plate cryo-electron tomography.
More...Complement Regulation
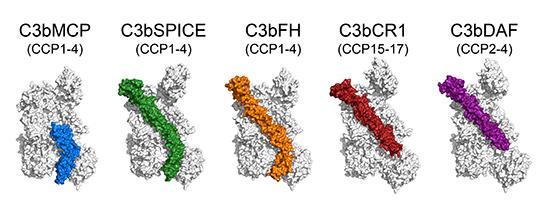
Regulators of complement activity mediate inhibitory mechanisms through common C3b-binding mode
More...Stem Cells
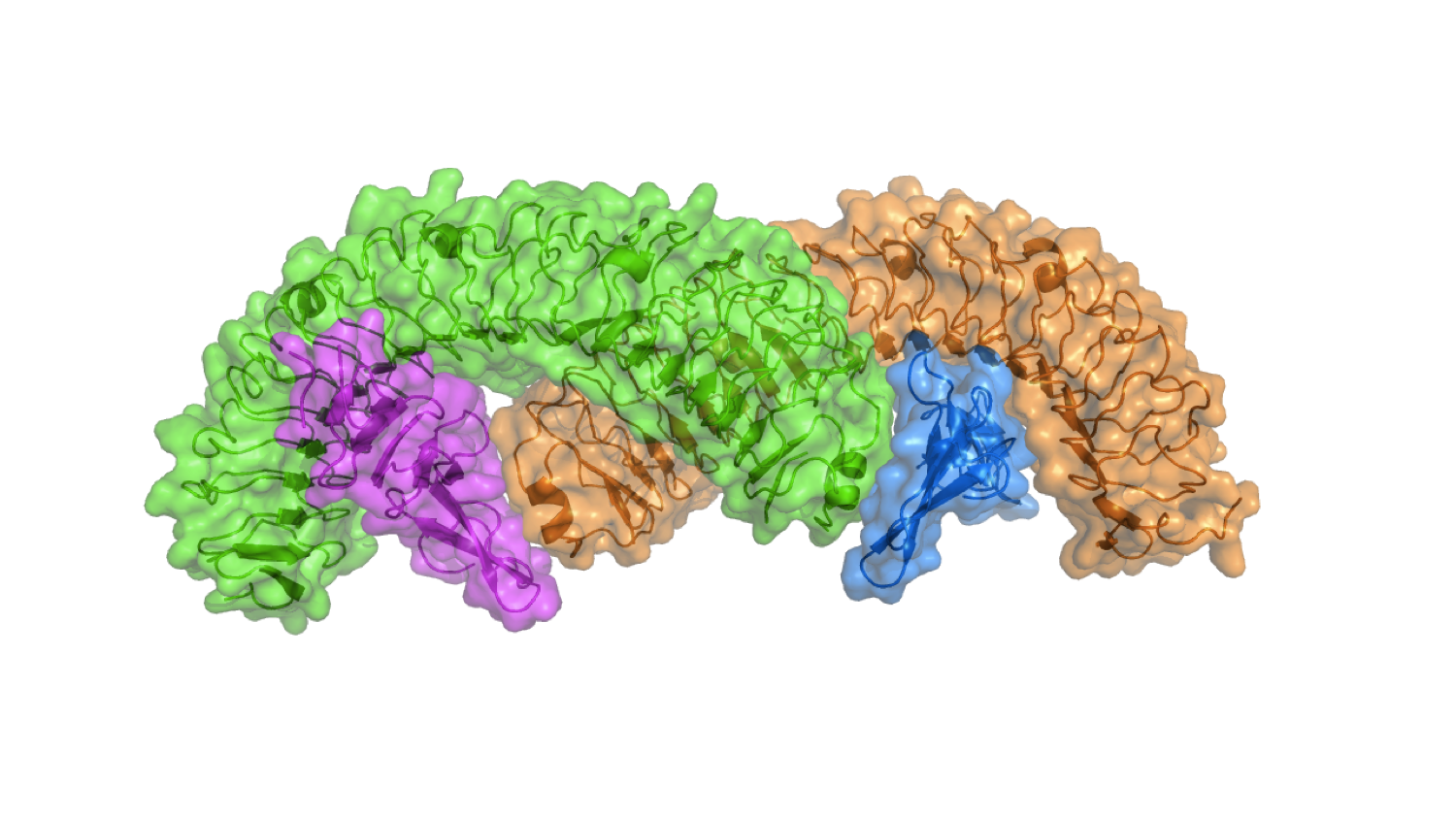
Stem cell growth factor R-spondin 1 in complex with the ectodomain of its receptor LGR5
Stem Cells
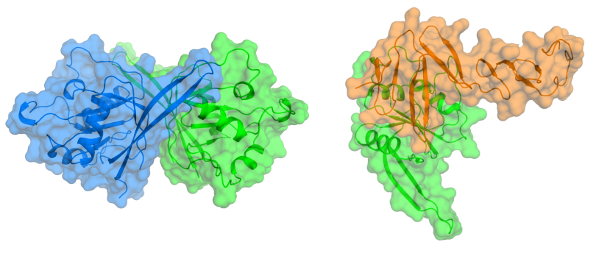
Structures of Wnt-antagonist ZNRF3 and its complex with stem cell growth factor R-spondin 1; implications for signaling